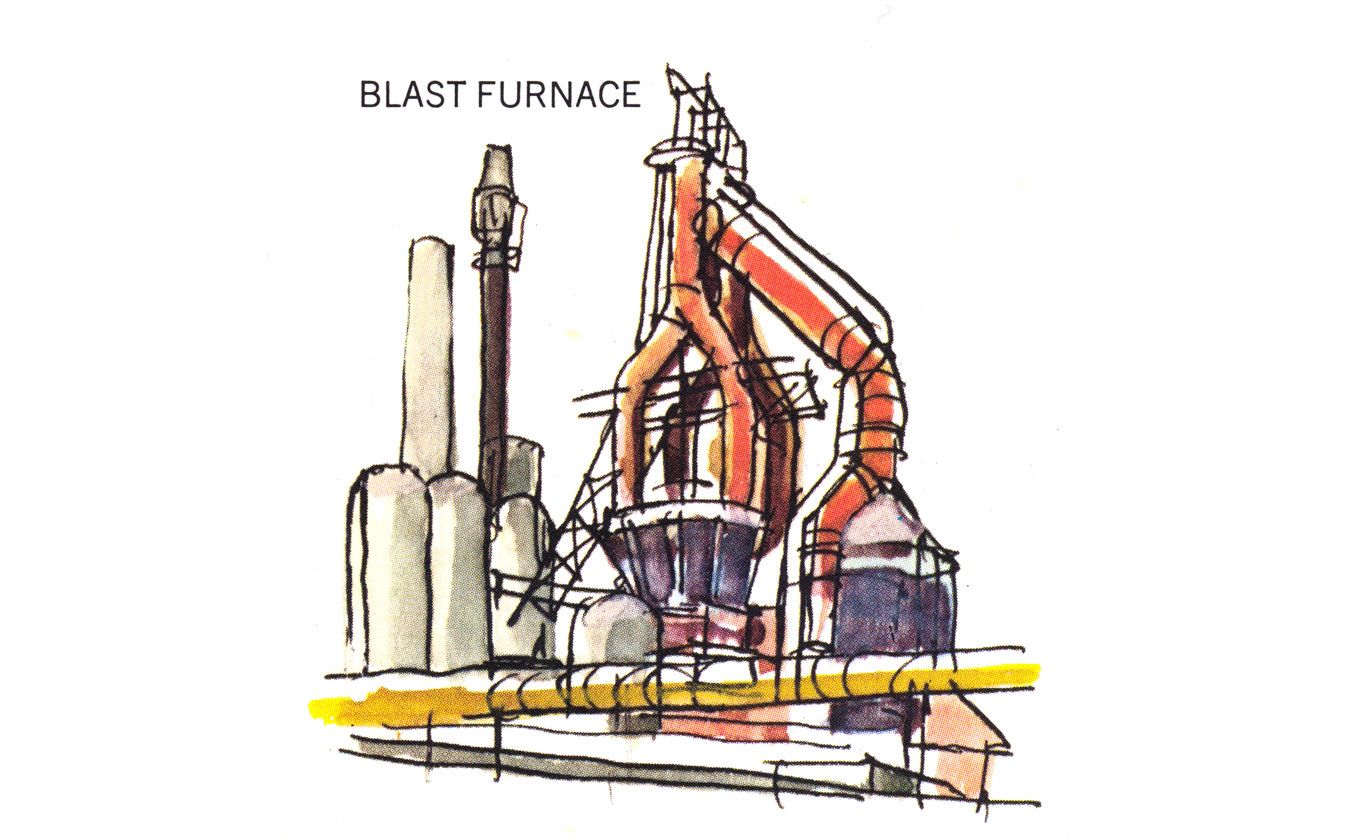
To convert iron ore into metallic iron, oxygen and impurities must be removed. This process requires heat and a reducing agent, a substance that combines with the oxygen and releases it from the ore.
In the blast furnace process, iron ore, coke (processed coal), and limestone (the charge) are poured into a vessel lined with refractory (heat-resistant brick). When heated, the coke reacts with oxygen and the limestone combines with impurities and removes them from the ore. What remains is liquid iron, which is then used to form a workable metal.
